Following the annual meeting of the implant intolerance work group, “AG11 Implantatunverträglichkeit” (DGOOC, German Society for Orthopaedics and Orthopaedic Surgery) at the german congress of orthopedic and trauma surgery (DKOU) in Berlin in 2016 the Histopathological Implant Register (“Histopathologisches Implantatregister”) was established.
2024 the work group implant safety (DGOU) was founded.
Its main aim is to provide a systematic, epidemiological data overview of joint prosthesis and implant pathologies in view of the entire histopathological spectrum, classified according to SLIM consensus classification into:
-
non-infectious (e.g. particle-induced)
-
infectious (including bacterial identification)
-
functional
-
immunological
-
neoplastic
Currently the data set (as of 2024) includes around 50.000 cases of joint prosthesis and implant pathologies. (ICD T84.0, T84.5, T84.9, T85, A49.8, M10, M11, M19, M86)
Image Gallery
Supramacro PE-particles in SLIM Type 1
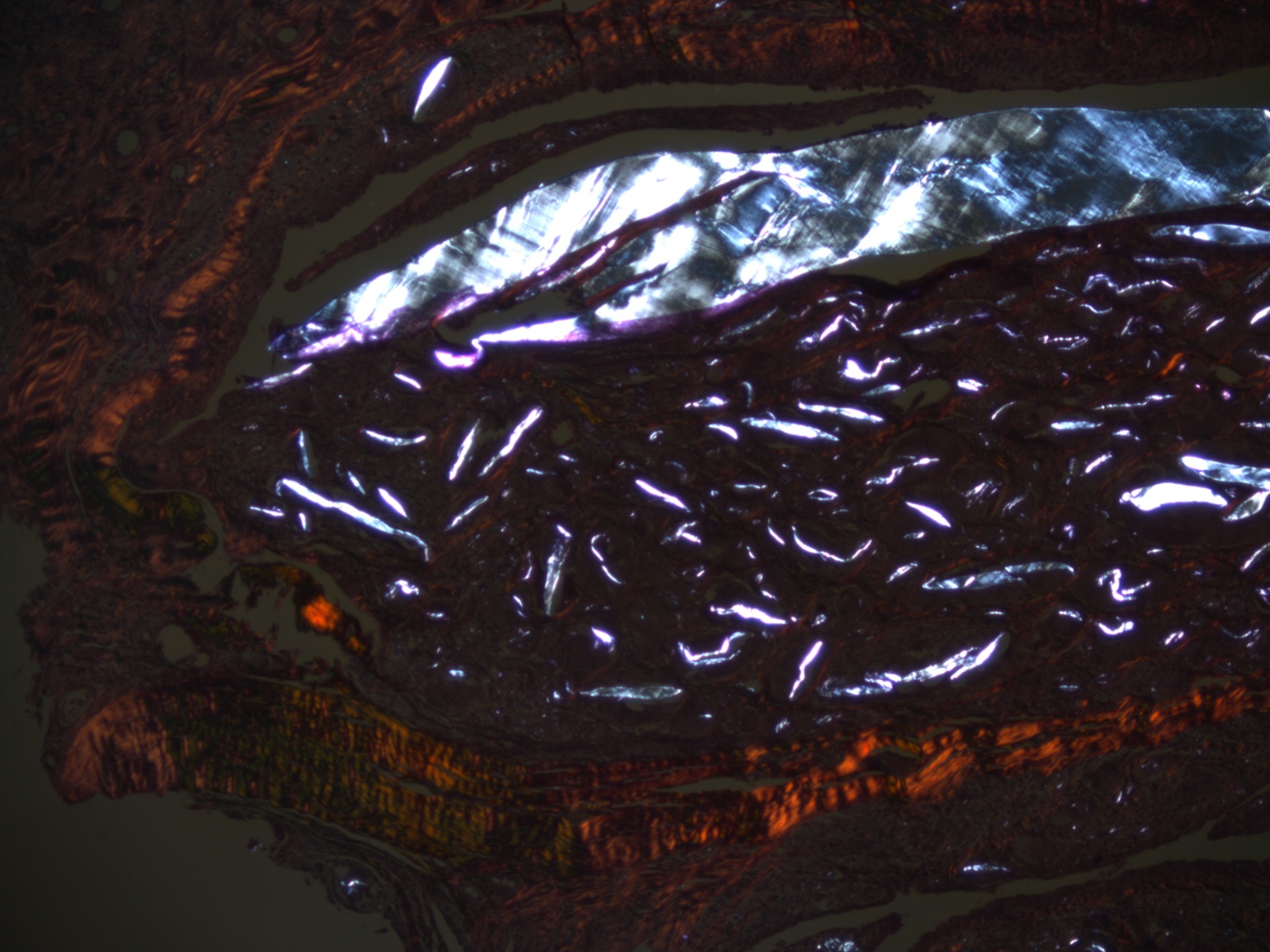
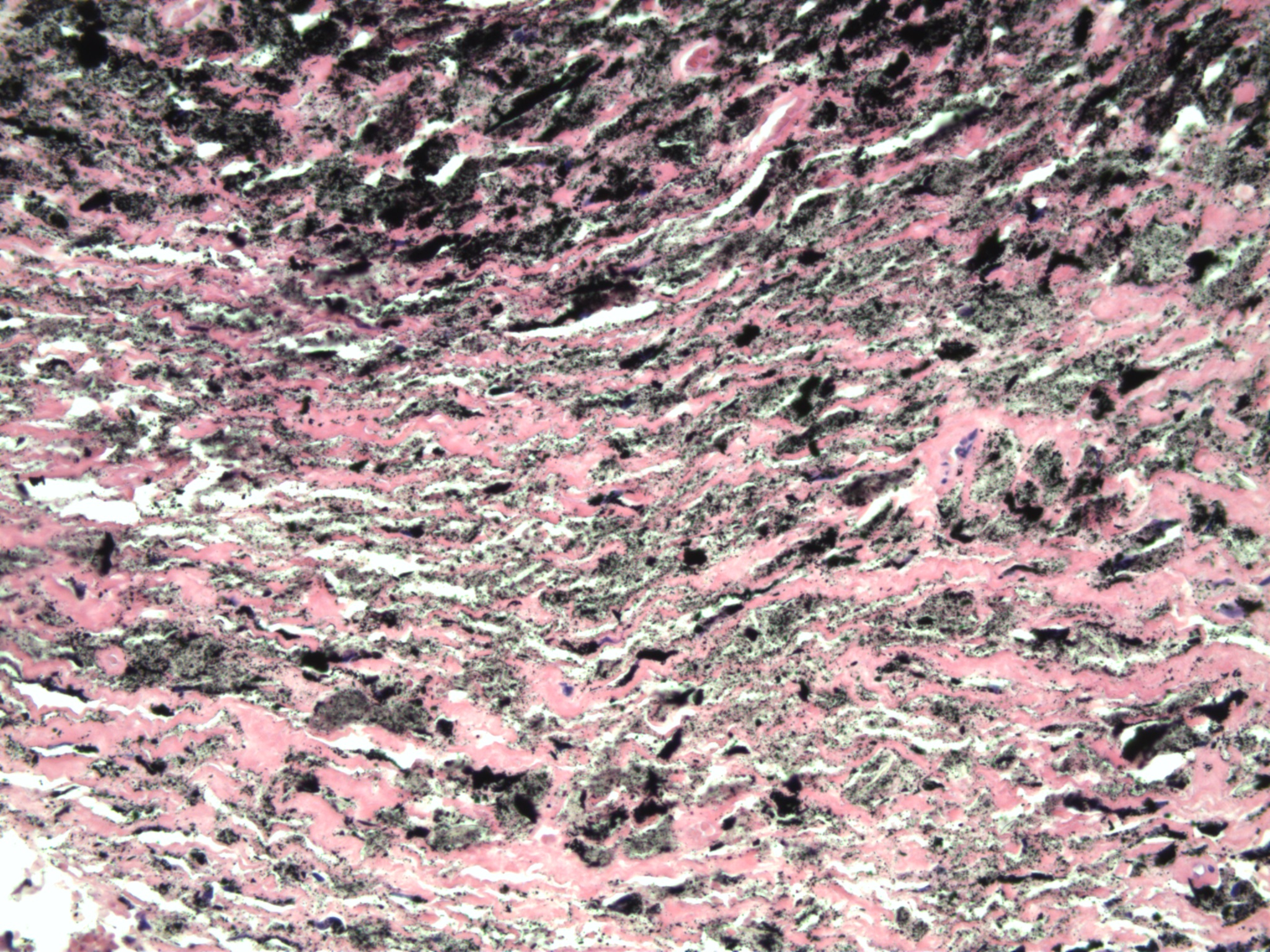
Microparticular titan particles in SLIM Type 1
Urate crystal deposits in gout
Micro, macro and supramacro silicone particles in SLIM Type 1
SLIM infectious Type 2, CD-15 Focus Score: 312 (CD15 Quantifier Mode, VmScope-Berlin.) microbiological diagnosis: Candida albicans
Microparticular and macroparticular ceramic particles in SLIM Type 1
 Deutsch
Deutsch en_US
en_US